 United States
United States United States
United StatesTo participate in this study, you or a loved one must:
Additional criteria for participation will be reviewed by the study team during your first appointment.
You will meet with a study coordinator at a study site location closest to you. You will learn more about the research study and the benefits and risks of participation.
The study coordinator or study doctor will review the Informed Consent Form with you. Before you make a decision about whether you want to participate in this study, you will have the chance to ask any questions about the details of the study and the Informed Consent Form. You will be asked to sign the form only if you are comfortable and willing to participate in the study. After you have given your consent, additional screening tests will be performed to make sure that you qualify to participate in the clinical study.
Efzofitimod was developed with the goal of resolving chronic lung inflammation, which may allow pulmonary sarcoidosis patients to reduce or eliminate the use of steroids while preserving lung function and improving physical symptoms. The goal of the EFZO-FIT™ study is to further test the safety and effectiveness of efzofitimod.
The study drug, efzofitimod, contains a protein that is thought to help to reduce the inflammation in the lungs which causes the lung damage and symptoms in pulmonary sarcoidosis.
After you have signed the Informed Consent Form, and the additional screening tests confirm that you are eligible to participate in the study, you will be enrolled as a participant and randomly assigned to a treatment group (to receive efzofitimod or placebo).
The planned length of this study is approximately one year. After the initial Screening Visit, you will be required to attend a total of 14 additional Study Visits at the study site closest to you. At the first 12 of these visits, scheduled once every 4 weeks, you will receive an intravenous (IV) infusion of either efzofitimod or placebo. A variety of standard and specific medical tests (such as a review of vital signs, ECG, and pulmonary function tests) will be performed at each Study Visit. The full list of all medical tests, exams, and procedures can be discussed with the site staff. Two weeks after each in-person study visit you will be contacted by telephone to review your progress.
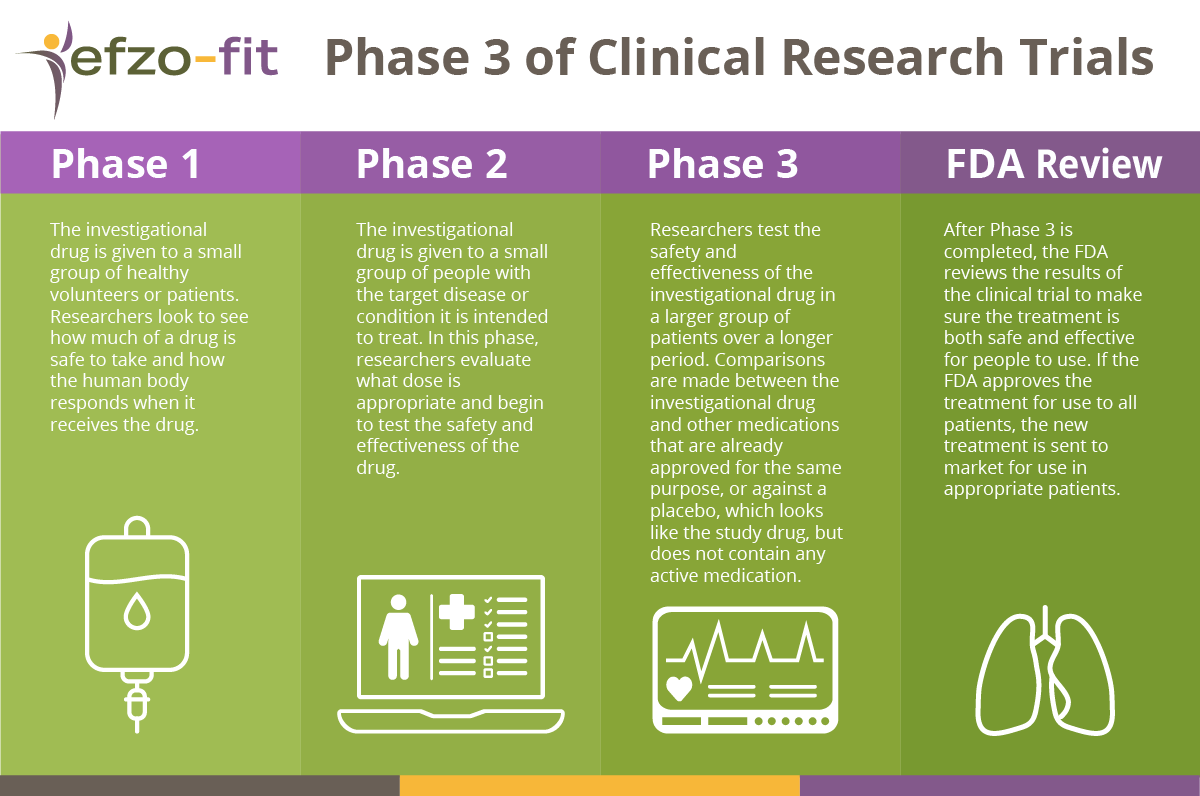
The EFZO-FIT study is in Phase 3 of clinical research trials. An investigational drug must pass three phases of clinical testing before it is approved by a country’s regulatory authority and made available to the public. The phases are as follows:
Phase 1: The investigational drug is given to a small group of healthy volunteers or patients. Researchers look to see how much of a drug is safe to take and how the human body responds when it receives the drug.
Phase 2: The investigational drug is given to a small group of people with the target disease or condition it is intended to treat. In this phase, researchers evaluate what dose is appropriate and begin to test the safety and effectiveness of the drug.
Phase 3: Researchers test the safety and effectiveness of the investigational drug in a larger group of patients over a longer period. Comparisons are made between the investigational drug and other medications that are already approved for the same purpose, or against a placebo, which looks like the study drug, but does not contain any active medication.
After Phase 3 is completed, the FDA reviews the results of the clinical trial to make sure the treatment is both safe and effective for people to use. If the FDA approves the treatment for use to all patients, the new treatment is sent to market for use in appropriate patients.
The study centers are located throughout the United States, Puerto Rico, the United Kingdom, Germany, the Netherlands, France, Italy, Spain, and Japan.
You do not have to stop taking your medication before participating in the study unless you are advised to do so by the study center staff or your primary care physician.
No, there will be no cost to you for the study therapy or study procedures. Travel reimbursement for the study visits may also be available.
No, health insurance is not a requirement to participate in this study.
Clinical studies, also known as clinical trials or research studies, are conducted by doctors and researchers to see if new medications or treatments are safe and effective. Clinical Studies must be completed, and the results carefully examined before a new medication can be approved for use by the general public. Participation in a clinical study is completely confidential and will be protected just like any other medical information.
Research studies are used to test new medications for safety, tolerability, and effectiveness before they are approved for use by the public.
You can find out more information about clinical studies by browsing through www.clinicaltrials.gov.This is an online US government database that is managed by the National Library of Medicine. It provides information about both federally and privately supported clinical research studies.
Additional information about sarcoidosis studies and other resources can be found at https://www.stopsarcoidosis.org/find-a-clinical-trial/
All research studies require that doctors and/or researchers give interested participants complete and accurate information about the risks, benefits, and activities of a research study. Interested participants will sign an Informed Consent Form before they enroll in the study. This form shows that a participant understands what will happen during the study and that they can leave the study at any time.
In certain types of clinical studies, some participants are given a placebo instead of the experimental drug: a placebo looks like the experimental drug but does not contain any medicine. Both the participant and the researcher are ‘blinded’, which means that they do not know during the study which participants are getting the experimental drug. This allows the researchers to compare the results seen in participants who received the experimental drug with the results seen in those participants who did not receive the experimental drug.